Literature Review on DNA Vaccines Coupled with Electroporation
Bryan GerberWhen a virus infects a cell, it presents antigens, or foreign markers, on the surface of the cell. These antigens are molecules that provoke an immune response. When an antigen is presented, the body has two possible courses of action, the humoral and cell mediated responses. In the humoral response, B cells attach to the antigen and then helper T cells (CD4+) attach to the B cell. This causes the B cell to differentiate into a plasma cell, which can release antibodies or a memory B cells which stay in the bloodstream. These memory B cells are used later on if infection reoccurs, which is the basis of vaccination. In a cell mediated immune response antigen presenting cells (APCs) attach to an antigen and “present” a copy of it and send out interleukins (signals) to cytotoxic T cells / killer T cells (CD8+) to target and kill cells with said antigen. Helper T cells (CD4+) are used in both responses and help to dictate which response is used.
In the early 1990’s D. C. Tang, M. DeVit, and S. A. Johnston first introduced the concept of DNA vaccines. This is a form of vaccination where foreign plasmid DNA encoding for a certain antigen is injected into cells. These cells then carry out the translation and transcription necessary to present the given antigen. Such research entities as the NIH and pharmaceutical companies such as Merck are interested in this vaccination since the induced immune responses can be theoretically used to fight diseases such as HIV, malaria, HCV, HBV, Anthrax, influenza, and cancer [1,3,4,6]. The Department of Defense has invested in DNA vaccine research as well because it believes the technology may also lead to a quick, nationally administered cure in the case of bioterrorist weapons [2]. One problem confronted with this field is the low rate of uptake when naked DNA is simply injected into large animals. Such a problem is overcome with the process known as electroporation, which is the directed use of electric fields to create transient pores in cellular membranes, which allows for uptake of foreign material such as DNA.
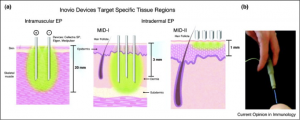
There are different methods of administering DNA vaccines with electroporation; mainly they are intramuscular (IM) injections or intradermal (ID) injections. Intramuscular injections usually involve injections in the thigh area with electrodes inserted 1 cm to 2 cm into the muscle [1,6] while intradermal injections involve injection and accompanying electrode insertion 1 to 3 mm into the skin [6]. The literature review “Electroporation delivery of DNA vaccines: prospects for success” provides a helpful illustration of devices made by Inovio, a pharmaceutical company, for these two methods (Figure 1) [6]. In a study called “Enhancement of DNA vaccine potency in rhesus macaques by electroporation,” performed by Otten at a lab ran by a biotechnology company, Chiron Corporation, it was found that taking the IM route lead to both increased cell-mediated immune response (such as killer T cells) and humoral response (antibodies). In general there was an increase in helper T cells, killer T cells, and antibodies found in the electroporated group than the non-electroporated group [1]. This suggests that electroporation increases intake of plasmid DNA, a result attributed to increased cell membrane permeability and DNA intake through an electrophoretic process [5].
Figure 1. The above pictures are graphical illustrations of electroporation devices inserted into the body for IM and ID (labeled MID for minimally invasive devices) routes. The number of electrodes can be varied based upon devise design. Figure from “Electroporation delivery of DNA vaccines: prospects for success.”
Lauren Hirao and her team at the Department of Pathology and Laboratory Medicine at the University of Pennsylvania School of Medicine carried out a similar study in which IM and ID injections were compared and contrasted. It was reported that the IM route had higher IFN-γ responses (a signaling molecule used in both cellular and humoral mediated responses), while the ID route had better CD4+ (helper T) and CD8+ (cytotoxic T cell) responses. In addition it was found that the ID route had a 5-fold higher increase in humoral response than the IM route. It should be noted that the above test had different pulsing parameters for the IM and ID routes [2]. This is of particular concern when considering the typical controlling environment of a well-designed study. Increased electric field strength results in a combination of increased uptake of exogenous materials as well as decreased cell viability. This could directly influence results considering increased DNA uptake would lead to more cells presenting the given antigens, while decreased cell viability would lead to less cells presenting the given antigens. Generally, optimal field strengths are found where these two variables are balanced, but these electroporation parameters differ based on the cell. Although Hirao’s study did use different pulsing parameters for the IM and ID route, this may be due to the fact that different cells were targeted (muscle versus skin). As a consequence, different parameters may have been optimal for the different cell types and therefore one common set of parameters could not be used.
Considering the IM routes and ID routes seem to have their respective strengths, Mann recently (2014) carried out a study at Imperial College London called “Enhanced Immunogenicity of an HIV-1 DNA Vaccine Delivered with Electroporation via Combined Intramuscular and Intradermal Routes” which attempted to combine the two routes to see if it would result in any stronger immune response. The thought was that muscular tissues have less APCs but are easier to transfect (have express foreign DNA) while skin has an abundance of APCs but is harder to transfect; used in conjunction the overall immune response throughout the body would theoretically be greater. In contrast to Hirao’s study, which found that the IM route had higher IFN-γ responses [2], this study found that there was no statistical difference between the IFN-γ responses in the IM and ID groups. It did show that there was a higher antibody avinity (overall strength of affinities of multiple individual binding interactions) when IM and ID were used together [3].
The studies performed by Otten, Hirao, and Mann raise a question, which route is more successful at eliciting a useful immune response? And on top of this, can one be more successful than the other, considering manipulating electroporation parameters can increase or decrease success of DNA transfection? Decisions may have to be made on device and route specific cases. For example, Otten used an electroporation device that was composed of a 6-needle array with a 1-inch diameter and 1 inch needle length (from Genetronics, Inc.), Hirao used a device known as CELLECTRA 2000 (Inovio Pharmaceuticals Blue Bell), and Mann used both 5-mm and tweezer electrodes attached to an ECM 830 square-wave electroporation system (BTX). The wide variety of electroporation devices, with their varying electrode arrays and electric field wave generators, simply means that for each device the optimal pulsing parameters will have to be found. Concerning IM versus ID route, both may have to be tried for any given DNA vaccine to see which one elicits a stronger immune response.
Some concerns considering safety of electroporation enhanced DNA vaccines have been raised. Otten’s paper “Enhancement of DNA vaccine potency in rhesus macaques by electroporation” raises the issue of pain response in humans due to the invasiveness of some electrode designs [1]. All of the previously mentioned cases were performed on animals, but in a Phase 1 clinical study carried out by Diaz in 2013, humans were used as test subjects and were able to self-report pain as well as adverse effects (AEs). On average pain from just electroporation was ranked as a 2 (out of 10) and all AEs from the DNA vaccine where in the range of 1 to 2 (on a scale of 1 to 10) [4]. This suggests that DNA vaccines are safe. Many of the researchers in this study were Merck employees or owned stock in Merck. Merck is one of the worlds largest research driven pharmaceutical companies, so its employees may have personal interests concerning DNA vaccines. Considering the stability of DNA at room temperatures, it is not outside of questioning that a pharmaceutical company would pursue mass production of certain DNA strands if DNA vaccines were medically used on a large scale. In general, plasmid based DNA vaccines are easier to prepare, biochemically simpler, and safer than viral vaccines [5]. Therefore there may be some bias in this paper leaning results that favor DNA vaccine use and safety.
One other concern raised by Otten was the integration of plasmid DNA into host genomes [1]. Wang addressed this problem in his paper “Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation.” Following electroporation with foreign (in this case plasmid) DNA, it was found that the non-electroporated group had only 17 plasmid copies/ g of DNA (negligible) in the host DNA while the electroporated group had 980m copies/ g of DNA. This suggests that more of the foreign plasmid DNA had been integrated into the host’s genome following electroporation than when there was no electroporation. Integration means that part of the plasmid DNA was physically incorporated into the host genome as opposed to floating around extrachromosomal. Although the level of foreign DNA integration was increased, a worse case scenario of 1000 copies of foreign plasmid DNA per g of DNA is still less than the natural rate of spontaneous mutations that inactivates genes [5]. It is therefore suggested that the benefits of DNA vaccines enhanced by electroporation, such as possible immunity from diseases, may outweigh the possible consequence of integration. This research was performed at a Merck research laboratory, so it is similar to the Phase 1 studied carried out by Diaz in that the analysis of results may have had some bias.
There are still more questions to be answered in this field of DNA vaccines and electroporation. ID and IM routes have been shown to be successful in their own ways, but which method is more successful at vaccination against a disease such as HIV? In addition, it needs to be determined whether there are any limitations to the methods of vaccination. Although many of the above experiments addressed increases in humoral and cellular responses, do we actually know if immunity was achieved? More tests on humans need to be performed to ensure that the vaccinations actually work.
Currently, vaccines commonly consist of parts of dead viruses or live but attenuated (weakened) viruses that provoke immune responses. One problem is that scientists still have not been able to create effective vaccines using the common techniques for diseases like HIV/AIDS and Malaria, and vaccination is too expensive for use in many poorer countries. If DNA vaccines aided by electroporation are proven to effectively cause immunization, vaccinations for some lethal diseases may become available. Although questions do exist, the future for DNA vaccines aided by electroporation appears promising.
Works Cited
- Otten, Gillis, et al. Enhancement of DNA Vaccine Potency in Rhesus Macaques by Electroporation. Vaccine. June 2004; 22(19): 2489-2493.
- Hirao, Lauren, et al. Multivalent Smallpox DNA Vaccine Delivered by Intradermal Electroporation Drives Protective Immunity in Nonhuman Primates Against Lethal Monkeypox Challenge. The Journal of Infectious Diseases. January 2011; 203(1): 95-102.
- Mann, Jamie, et al. Enhanced Immunogenicity of an HIV-1 DNA Vaccine Delivered with Electroporation via Combined Intramuscular and Intradermal Routes. April 2014; 88(12): 6959-6969.
- Diaz, Claudia, et al. Phase 1 studies of the safety and immunogenicity of electroporated HER2/CEA DNA vaccine followed by adenoviral boost immunization in patients with solid tumors. Journal of Translational Medicine. 2013; 11(62).
- Wang, Z., et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Therapy. 2004; 11: 711-721.
- Sardesai, Niranjan and Weiner, David. Electroporation delivery of DNA vaccines: prospects for success. Current Opinion in Immunology. June 2011; 23(3): 421-429.